Thing called metal, a typical example of which is the iron is very sturdy and hard.
However, no matter how sturdy, there are natural enemies. One of them is the "rust".
The "rust" is what metal is changed to another substance by undergoing a chemical reaction with oxygen(O2) and moisture(H2O) in the air and "oxidized".
Red rust of iron in a typical example, is what iron is changed to iron oxide (Ⅲ).
Since the iron oxide (Ⅲ) is a very fragile material, leave and the iron will to tatters.
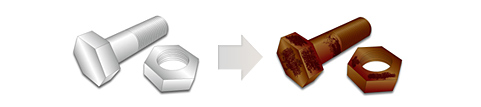
The rust prevention, in order to prevent the "oxidation" of such a metal, is to cut off contact between the metal and the environment.
To that end, in the painting or plating, covered with a film on the surface of the metal, it is the metal'll be protected so as not to come into contact with the surrounding environment directly.
*What is "Oxidation"?
The original definition of Oxidation was "that the material and the oxygen is linked."
"Iron oxide" is a substance that iron and oxygen has changed ties.
The current definition of it, is to be taken away is "electronic" some from the "atoms" that make up the substance.
Metal is easily generally taken the "electronic", there is likely to nature or "oxidation".
"Chromium" is one of the metal elements, there is a feature that it is difficult to rust.
Taking advantage of the feature of this rust resistant, chromium had been used to as a "Chromium plating", or a post-processing of zinc-plated "Chromate treatment".
Had been primarily used for the chromate treatment is hexavalent chromium compounds are presently regulated performed as toxic.
The hexavalent chromium compounds, has a very strong toxicity, also because there is also a carcinogen, using hexavalent chromium is currently is restricted in the world.
The trouble began regulation of hexavalent chromium, ever it is the industry such as an automobile, which has been using the hexavalent chromium to "chromate treatment".
The current alternative, trivalent chromium compound (There is no toxicity unlike hexavalent) are used, but there are parts of fall than hexavalent in terms of performance, etc., and because it is the same as hexavalent chromium compounds, the risk of depending on use environment changes to a harmful hexavalent from harmless trivalent is has been reported.
Therefore, we raised the argument that attempt to limit the be used for trivalent chromium compounds, we believe that the future becomes the direction in which the plating process using a chromium compound will be limited.
The anti-corrosion surface treatment agent ZECCOAT what the Company developed, does not use chromium, is intended to meet the demands of such era.
Trivalent chromium will still be used for a while, but the era of the flow is proceeding in the direction of eliminating the chromium using, the era of the chromium-free(non-chrome) would come sooner or later.